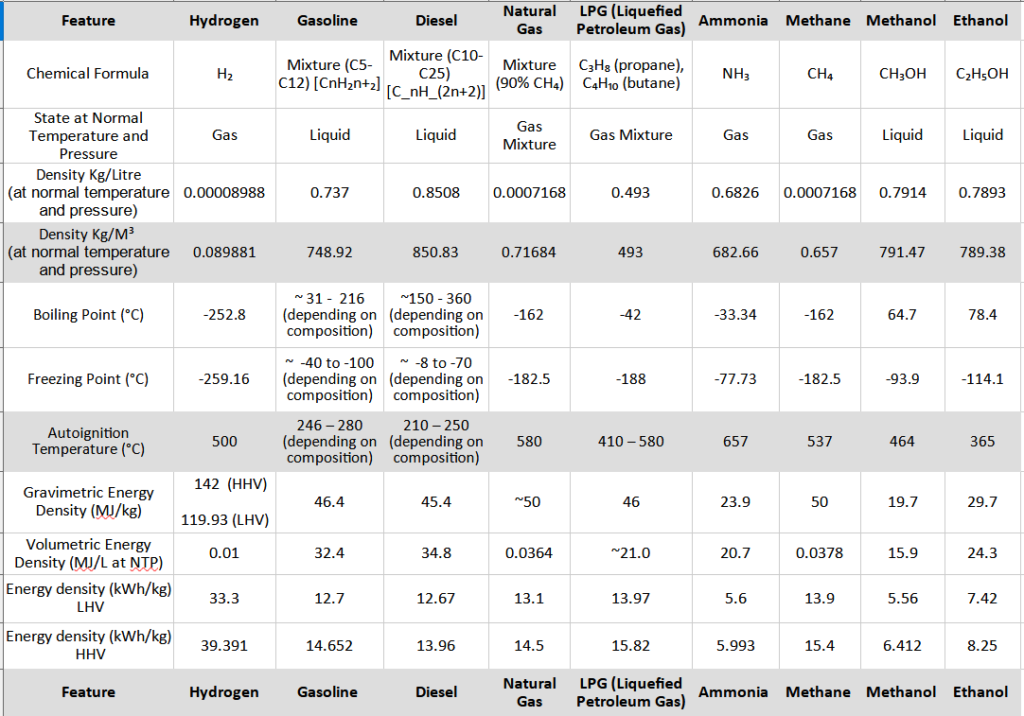
Hydrogen is compared with other key fuels like Gasoline, Diesel, Natural Gas, LPG (Liquefied Petroleum Gas) Ammonia, Methane, Methanol, and Ethanol.
The below table highlights the essential, basic characteristics of hydrogen and compare the same with fossil fuels, natural gas, ammonia, methane, methanol, and ethanol.
Hydrogen density is a physical property that defines how much mass of hydrogen is concentrated within a specific space or volume. Essentially, it tells us how tightly packed the hydrogen atoms or molecules are in a given space. We express density using units in Kg/Litre or Kg per cubic meter (kg/m³).
Being the lightest among all the elements, gases, if you consider the density in kg/L, only .000089 kg of hydrogen is present in 1 Litre where as for gasoline it is 0.7 kg, and for diesel 0.85 kg and 0.789 for ethanol. This inherent characteristic of hydrogen translates to a lower mass for a given amount of energy stored compared to other denser fuels. However this has a huge drawback while storing and transporting. Hence under normal conditions, too much space is required to store hydrogen gas. Thus hydrogen is virtually not stored or transported in gaseous form at atmospheric pressure because it is highly inefficient.
Another very important physical property is energy density, which can be measured in two ways: gravimetric and volumetric. Hydrogen has highest gravimetric energy density (see the table) being the primary reason for considering hydrogen as a strong contender, as an alternative among all types of fuels (excluding fossil fuels). In simple terms, By weight, hydrogen holds a highest amount of energy. This physical aspect makes hydrogen as an energy carrier as well. However If you take volumetric energy density, hydrogen is the lowest among all.
This article is Copyright protected






Add comment